Isabelle Cheng
Block 2-2
Chem 11 Ms. Chen
The Lewis Structure involves using dots. It is very simple and easy to understand!
Here are some examples!
| Number of Valence Electrons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Example | Hydrogen | Group I (Alkali metals) | Helium | Group II (alkali earth metals) | Group III | Group IV | Group V | Group VI | Group VII (Halogens) | Group VIII except Helium (Noble Gases) |
| Lewis Structure (electron dot diagram) | 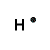 | 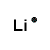 |  | 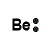 | 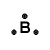 | 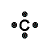 | 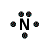 |  | 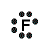 |  |
That is what Lewis structures look like and how the dots are formed with the symbol in the middle to identify which element it is. The purpose of the Lewis Structure is that it shows the valence electrons that are distributed in a molecule. Also there is a rule called the octet rule which is saying that most of the atoms want the electrons to form covalent bonds. This rule does not necessarily apply to all of the atoms.
Here is an example of H20!

Here are some practice ones!
H
● ●
NH4 + = H ● N ● H
● ●
● ●
H
The first step is to first draw 4 H’s because the element is H4.
Then you write a N in the middle with the surrounding H’s because it is the only element and it only has one.
The third step is to then daw two dots for every H surrounding the N.
You apply the same rules and the same procedure for the other elements.
Then that helps you get the Lewis Structure!
No comments:
Post a Comment