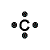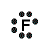What are functional groups?
-organic compounds that are comprised elements other than carbon or hydrogen (ex. nitrogen & oxygen)
-tend to be the most reactive part of the molecule
types of functional groups: alcohols, halides, aldehydes, ketones
Halides & Nitro Compounds
-naming: close to that of simple hydrocarbons - can be fixed to alkanes, alkenes, & alkynes (end w/-ANE)
-if there is more than 1 of the SAME halogen/molecule attached to 1 molecule, add the prefix di, tri, tetra, penta, etc. before the halogen/molecule prefix
-if there are DIFFERENT halogens/molecules attached to 1 molecule, list the positions in alphabetical order
(ex. 2,4,4-tribromo-5-chlorononane)
-the main chain name obtains one of these prefixes that are fixed to it
| Halogens | Molecules | ||
| F | Fluoro | NO2 | Nitro |
| Cl | Chloro | ||
| Br | Bromo | ||
| I | Iodo |

| Ex. 1 (top left): # of carbon atoms: 1 à methane # of halogens: 3 Cl in the first carbon position à tricloro | Ex. 2 (top right): # of carbon atoms: 1 à methane # of halogens: 4 Cl in the first carbon position à tetrachloro |
Ex. 3 (below):

***Note: each empty corner represents ONE carbon atom, thus there are 2 carbons in this molecule! :D
2 carbons = ethane
1 Cl attached in the first carbon position (recall: there isn’t a set direction in which to count the position that the carbon is in; count from the whichever way creates a smaller #, in this case, from left to right)
This compound is called 1-chloroethane or simply chloroethane.
Properties of Halides
-tend to be insoluble in H2O
-compounds combined with fluorine are non-reactive (inert)
Ex. Teflon, which makes up the thin non-stick surface on frying pans
-compounds containing chlorine or bromine are more reactive under extreme conditions
-compounds that have iodine are quite reactive; they can be easily substituted with other functional groups
Properties of Nitro Compounds
-often insoluble in H2O
-inert to chemical attack unless under extreme conditions
-generally explosive
Ex. TNT (trinitrotoluene & nitroglycerine)
-most smell pleasantly fruity
Alcohols
-have an –OH (hydroxyl) functional group
-naming: use the longest carbon chain containing the OH group & replace the ‘e’ ending in the parent hydrocarbon with ‘ol’
-remember: always use the smallest # possible for the OH group even if the branched parts will get a higher # (ex. 4-methyl-2-pentanol rather than 2-methyl-4-pentanol)
-if a molecule contains 2 or more hydroxyl groups, # both and add –diol, -triol, etc. endings
 ß 1, 2-ethanediol
ß 1, 2-ethanediolEx. butanol

-the longest carbon chain containing OH is 4 à butanol
Properties of Alcohol
-somewhat soluble in H2O: the hydroxyl (OH) group breaks off, but the hydrocarbon chain is often insoluble
-ALL alcohols are poisonous to an extent
Ex. Ethanol: type of alcohol found in alcoholic beverages
Ex. 

Longest carbon chain w/OH: 3 à propanol
Some other types of alcohols:

Aldehydes
-have a double bonded oxygen at the end of the chain
-follow regular naming rules & change parent chain ending to –al (do NOT confuse w/alcohols, even though they are pronounced the same)
 butanol
butanol propanal
propanal 2-methyl propanol
2-methyl propanolKetones
-contain a double bonded oxygen that is NOT on either end of the hydrocarbon chain
-ending: -one
 hexone
hexone
 acetone
acetone hexanone
hexanoneProperties of Aldehydes & Ketones
-both: somewhat soluble in H2O
-aldehydes: quite reactive; easily turned or ‘oxidized’ into carboxylic acids
-ketones: rather non-reactive

Youtube links for extra help:
http://www.youtube.com/watch?v=ndOwa5zET8Y (VERY helpful)
http://www.youtube.com/watch?v=4rRA6fuS_JU (hahahahhahaha)