Alkenes, Alkynes, who cares? They're the same thing.
WRONG.
Even though they both form bonds that contain fewer Hydrogen atoms attached to Carbon atoms, THEY ARE VERY DIFFERENT!
Naming these compounds are relatively easy, as they follow the same rules as naming Alkanes. Just remember to change the ENDING to the appropriate one!
Let's start with Alkenes.
Alkenes-->contain one or more double bonds
-->classified as unsaturated hydrocarbons
-->CHANGE ENDING TO "-ENE"
eg. CH3-CH2-C=CH-CH3
|
CH3-CH3-CH3
2-propyl-2-pentene
*notice how the ending of the parent chain changed
Alkenes have a many possible structures (same), BUT have different geometrical shape
-->this is special to ONLY ALKENES called geometric insomers
Geometric insomers-->occur when double bond is formed, having side chains attached (top & bottom)
-->2 types:
-trans: (top&bottom, bottom&top)
-cis: same side (top&top, bottom&bottom)
So, practice what you've learned!
Draw these structures:
4-methyl-2-pentene
3-propyl-2-heptene
Now, for the Alkynes!
Alkynes-->contain one or more triple bonds (yes, now you use 3 LINES!!)
-->also unsaturated hydrocarbons
-->naming rules are still the same, but CHANGE ENDINGS to "-YNE"
eg. CH3-CH-CH3-C(insert triple bond here)C-CH3
| |
C CH3
5,6-methyl-2-heptyne
It's simple...REALLY!
Make sure you do some extra practice from the textbook!!
Memorize all the prefixes (it'll help)!
Jialynn.
Tuesday, May 31, 2011
Thursday, May 26, 2011
Organic Chemistry - May 26, 2011 - Isabelle Cheng
Isabelle Cheng
Block 2-2
Chemistry 11 Ms. Chen
Organic Chemistry
What is organic chemistry anyway?.....
Organic Chemistry:
introduction to the diverse chemistry of carbon
scientific study of the structure of carbon-based compounds and hydrocarbon
can vary from small to large
has a specific naming system
different kinds of structures to represent a molecular formula
form chains of carbon atoms
Properties:

A carbon that has 4 bonds

A carbon atom that has 4 hydrogen atoms

A carbon-carbon bond

Branched Chain

Rings
Naming:
There are certain names for each molecular formula!
1 - meth
2 - eth
3 - prop
4 - but
5 - pent
6 - hex
7 - hept
8 - oct
9 - non
10 - dec
You use these prefixes in the beginning and the endings for alkanes is “-ane.”
The formula is CnH2n+2, if you plug in the numbers and it doesn’t work then that means that it is not from the alkane group!
When looking at the structures: Always look at the longest chain and write the molecular formula from there!
For example:

It is called Propane because there are three of the longest chain.
For the Alkayl group, they have a different formula and different naming system:
The ending is “-yl,” so an example would be methyl.
For example:

This structure is called 3-methyl-pentane because the bond is on the third position and it is called methyl because it is from the alkyl group. It is called pentane because the longest chain has 5 of them.
Extra facts: The sum of all valence e- of each atom in the molecular formula must equal the number of electrons in your Lewis Dot Structure.
Except for a few elements, ex. H, most elements want a full valence shell.
Sunday, May 22, 2011
chemical bonding (by: mandy)
CHEMICAL BONDING
There are 3 types of chemical bondings: Ionic Bonding, Nonpolor Covalent Bonding, and Polar Covalent Bonding.
Nonpolor Covalent Bonding: electrons are shared equally
Polar Covalent Bonding: electrons are shared unequally
Ionic Bonding: electrons are transferred between two atoms
Electrostatic Force
Electronegativity: the measure of the ability of an atom to attract electron from other atoms.
Metals have LOW electronegativity
Non-metals have HIGH electronegativity
*The greater the difference of electronegativity, it is more likely to be Ionic Bond
ENeg Diff. = | ENeg1- ENeg2 |
ENeg Diff. <0.5 (non-polar covalent)
ENeg Diff. 0.5 to 1.8 (polar covalent)
ENeg Diff. >1.8 (ionic)
London Forces
Polar Covalent Bonds
A molecule is polar if there is an imbalance with electrical charge. [This is called: Polarity]
Atoms with higher electronegativity (delta -)
Atoms with lower electronegativity (delta +)
For example:
Hydrogen has an electronegativity of 2.1
Oxygen has an electronegativity of 3.5
therefore, O, with the higher electronegativity is labelled delta- and two H are labelled delta+
Arrow should be pointing at the negative atom.
study hard!
There are 3 types of chemical bondings: Ionic Bonding, Nonpolor Covalent Bonding, and Polar Covalent Bonding.
Nonpolor Covalent Bonding: electrons are shared equally
Polar Covalent Bonding: electrons are shared unequally
Ionic Bonding: electrons are transferred between two atoms
Electrostatic Force
Electronegativity: the measure of the ability of an atom to attract electron from other atoms.
Metals have LOW electronegativity
Non-metals have HIGH electronegativity
*The greater the difference of electronegativity, it is more likely to be Ionic Bond
ENeg Diff. = | ENeg1- ENeg2 |
ENeg Diff. <0.5 (non-polar covalent)
ENeg Diff. 0.5 to 1.8 (polar covalent)
ENeg Diff. >1.8 (ionic)
London Forces
Polar Covalent Bonds
A molecule is polar if there is an imbalance with electrical charge. [This is called: Polarity]
Atoms with higher electronegativity (delta -)
Atoms with lower electronegativity (delta +)
For example:
Hydrogen has an electronegativity of 2.1
Oxygen has an electronegativity of 3.5
therefore, O, with the higher electronegativity is labelled delta- and two H are labelled delta+
Arrow should be pointing at the negative atom.
study hard!
Sunday, May 15, 2011
Electron Dots and Lewis Structures - Isabelle Cheng
Isabelle Cheng
Block 2-2
Chem 11 Ms. Chen
The Lewis Structure involves using dots. It is very simple and easy to understand!
Here are some examples!
| Number of Valence Electrons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Example | Hydrogen | Group I (Alkali metals) | Helium | Group II (alkali earth metals) | Group III | Group IV | Group V | Group VI | Group VII (Halogens) | Group VIII except Helium (Noble Gases) |
| Lewis Structure (electron dot diagram) | 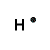 | 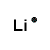 |  | 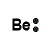 | 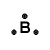 | 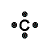 | 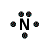 |  | 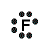 |  |
That is what Lewis structures look like and how the dots are formed with the symbol in the middle to identify which element it is. The purpose of the Lewis Structure is that it shows the valence electrons that are distributed in a molecule. Also there is a rule called the octet rule which is saying that most of the atoms want the electrons to form covalent bonds. This rule does not necessarily apply to all of the atoms.
Here is an example of H20!

Here are some practice ones!
H
● ●
NH4 + = H ● N ● H
● ●
● ●
H
The first step is to first draw 4 H’s because the element is H4.
Then you write a N in the middle with the surrounding H’s because it is the only element and it only has one.
The third step is to then daw two dots for every H surrounding the N.
You apply the same rules and the same procedure for the other elements.
Then that helps you get the Lewis Structure!
Monday, May 2, 2011
Periodic Table Familes and Trends.
#trending, teehee.
Who knew the periodic table created trends?
They're such fashionistas!
That was just for laughs...
ANYWAY, here's a video that explains everything. (:
Enjoy.
Who knew the periodic table created trends?
They're such fashionistas!
That was just for laughs...
ANYWAY, here's a video that explains everything. (:
Enjoy.
Subscribe to:
Comments (Atom)
